Integral solutions in regulatory compliance and operational efficiency.
We comprehensively manage all regulatory procedures before INVIMA related to medical devices and biomedical equipment, ensuring agile, precise processes aligned with current regulations.
We accompany organizations from the initial technical review to filing, follow-up, and final approval of each procedure, reducing times, rework, and rejection risks.
Manage procedure
We accompany organizations that seek to import medical devices and equipment throughout the regulatory compliance process required by INVIMA for CCAA certification.
We design and structure the required processes, train the personnel involved, and guide the company at every stage of the audit, ensuring a successful and smooth certification.

We support organizations that are close to their CCAA recertification through a complete internal audit that identifies non-conformities, risks, and improvement opportunities.
We implement necessary corrections, strengthen document management, and prepare the team to face the official visit with security, control, and total clarity.

We accompany organizations in full compliance with the requirements demanded by INVIMA for certification in Sanitary Conditions in manufacturing activities of biomedical devices and equipment.
We manage every stage of the process: regulatory interpretation, document structuring, process adequacy, personnel training, and preparation for the official inspection visit.
More information
We design, implement, and maintain management systems that remain updated, in permanent compliance, and ready for any surveillance visit or control audit.
We combine expert advice with AI tools that automate operational tasks, reduce administrative burden, and ensure continuous traceability of critical processes.
Through a comprehensive maintenance model, we ensure that your system always complies with current regulations, avoiding sanctions, findings, and regulatory delays.
Optimize your system
We accompany organizations throughout the implementation and certification process of ISO 9001 and ISO 13485 standards, ensuring solid, documented management systems aligned with international requirements.
We design and structure each process, train teams, and prepare the company to successfully pass the certification audit.
Our approach combines methodological clarity, document order, and intelligent tools that facilitate daily system management and ensure traceability.
Get certified with us
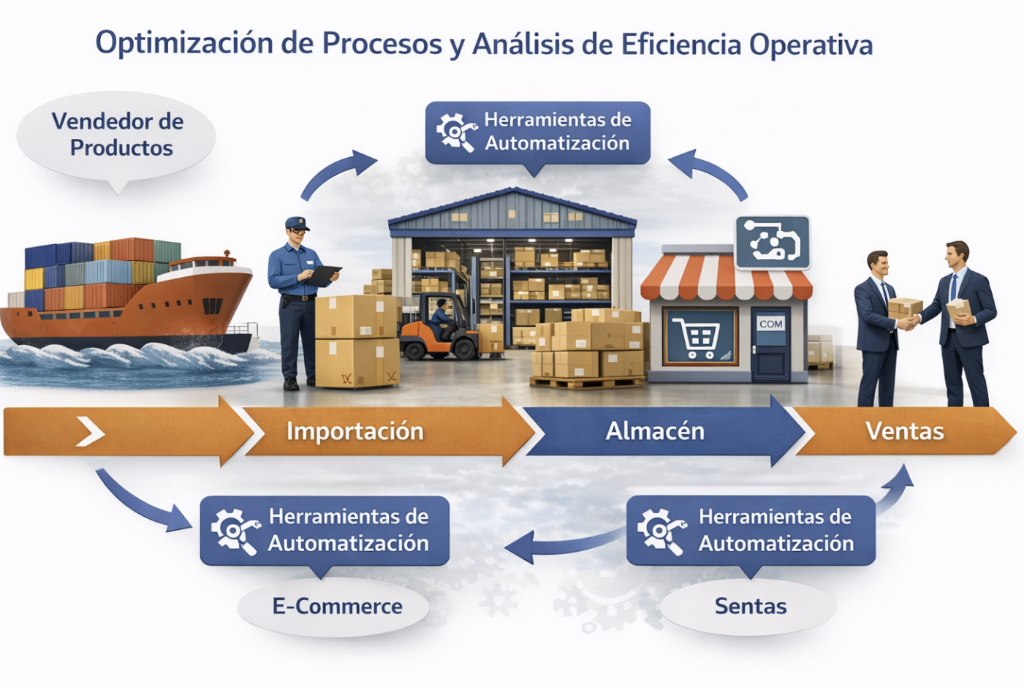
We help organizations identify real opportunities for improvement in their processes through a detailed lifting of the operation.
We analyze workflows, times, operational loads, and execution gaps to detect bottlenecks, rework, and non-value-added activities.
Based on this diagnosis, we evaluate options for automation, redesign, or simplification of processes, integrating modern tools —including AI, automation, and digital solutions— to reduce times, eliminate operational inefficiencies, and increase personnel productivity.
Improve your efficiency